Palladium-catalyzed carbonylative synthesis of benzofuran-2(3H)-ones from 2-hydroxybenzyl alcohols using formic acid as the CO source†
Abstract
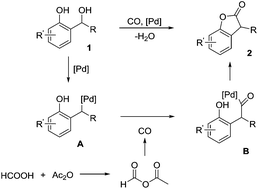
A palladium-catalyzed carbonylative intramolecular synthesis of benzofuran-2(3H)-ones from 2-hydroxybenzyl alcohols has been developed. In this procedure, formic acid was utilized as the CO source, and various benzofuran-2(3H)-one derivatives were obtained in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...