Zinc-catalyzed chemoselective alkylation of α-keto amides with 2-alkylazaarenes†
Abstract
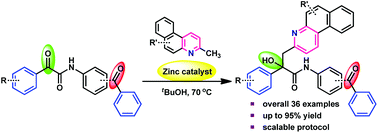
A zinc-catalyzed C(sp3)–H addition of 2-alkylazaarenes to α-keto amides to furnish azaarene incorporated α-hydroxy amides has been developed with a wide range of substrates in moderate to excellent yields, respectively. Chemoselective alkylation of the keto functionality of the α-keto amides in the presence of simple ketones is the key advantage of this Zn-catalyzed transformation. This approach has been demonstrated to one gram-scale synthesis. 1H NMR and D2O exchange experimental studies reveal that the reaction proceeds through a Zn-enamide intermediate.


 Please wait while we load your content...
Please wait while we load your content...