Dearomatisation approaches to spirocyclic dienones via the electrophilic activation of alkynes†
Abstract
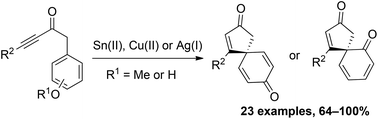
Two complementary dearomatising spirocyclisation protocols to generate spirocyclic dienones from anisole and phenol-tethered ynones are described, each proceeding via electrophilic alkyne activation. The first approach focuses on the spirocyclisation of para-substituted anisoles using either SnCl2·2H2O or Cu(OTf)2. The second approach, which enables the spirocyclisation of both ortho- and para-substituted phenols, uses silica-supported AgNO3 to generate similar scaffolds with much greater efficiency. Initial asymmetric studies are also outlined.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners


 Please wait while we load your content...
Please wait while we load your content...