Acylboranes: synthetic strategies and applications
Abstract
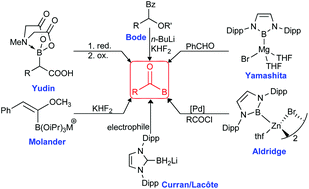
An overview of the synthesis and chemistry of acylborane compounds is presented. Acylboranes are a rare class of boron compounds, previously proposed as intermediates in several transformations and considered to be difficult to prepare. Methodologies for the preparation of acylborane compounds are based on both electrophilic and nucleophilic sources of boron. The former methods include addition of electrophilic boron reagents to acyl-anion equivalents, while the latter methods are based on boryl anion reagents which are trapped by electrophiles, such as aldehydes, diethyl carbonate and ethyl acetate. New methods to achieve acylboron-compounds based on oxidation of MIDA α-hydroxyboronates or α-bromomethyl alcohol are discussed. A one-step catalytic C–B coupling reaction for preparing acylboranes from palladium-catalyzed borylation of acyl chlorides using nucleophilic borylzinc reagents is included. Applications of acylboranes in chemoselective amide-bond forming reactions, converting them into functionalised boron derivatives are also discussed.


 Please wait while we load your content...
Please wait while we load your content...