Carbonylation as a novel method for the assembly of pyrazine based oligoamide alpha-helix mimetics†
Abstract
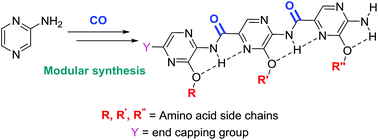
The design and synthesis of oligoamide α-helix peptidomimetics is reported. The oligoamide type systems are prepared in a modular fashion by coupling the monomers using palladium-catalyzed carbonylation chemistry. This enabled us to use substrates with a low nucleophilicity, leading to previously unreported pyrazine based oligoamide α-helix mimetics. The proof of principle is given by synthesizing a small set of compounds. Various end-capping groups were introduced and also a mixed multimer was successfully prepared.


 Please wait while we load your content...
Please wait while we load your content...