Access to functionalized thienopyridines via a reagent-capsule-assisted coupling, thiolation and cyclization cascade sequence†
Abstract
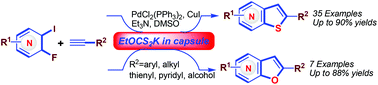
Thienopyridines and related heterocycles were prepared in a straightforward manner in moderate to good yields and under mild conditions by palladium-catalysed cross-coupling of ortho-fluorinated iodopyridines and terminal alkynes, followed by a reagent-capsule-assisted thiolation cyclization process. By applying paraffin wax capsules to prevent catalyst poisoning and undesired side reactions, the separation and purification processes were reduced.

 Please wait while we load your content...
Please wait while we load your content...