Sonogashira cross-coupling reactions of 3,5-dibromo-2,6-dichloropyridine†
Abstract
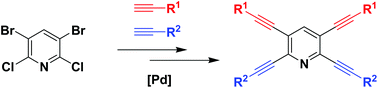
A new method for the chemoselective synthesis of alkynylpyridines from 3,5-dibromo-2,6-dichloropyridine has been developed. Optimized conditions give access to a variety of mono-, di-, tri- and tetraalkynylated pyridines in good yields. Interestingly, the employment of 3,5-dibromo-2,6-dichloropyridine as a starting material led to the opposite regioisomers of dialkynylated pyridines as compared to the application of 2,3,5,6-tetrachloropyridine.


 Please wait while we load your content...
Please wait while we load your content...