Stereoselective preparation of conjugated (Z)-1,3-enynes by dehydration reactions of allenic bromohydrins and the use of the enynes in base-mediated tandem allylation ene-carbocyclization reactions with β-ketoesters†
Abstract
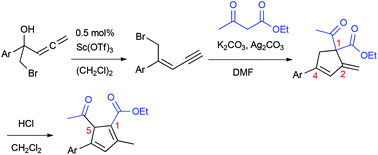
A procedure has been developed for the concise, stereoselective synthesis of (Z)-5-bromo-4-aryl-pent-3-en-1-ynes through Sc(OTf)3 catalyzed dehydration reactions of allenic bromohydrins. (Z)-1,3-Enynes are transformed to methylenecyclopentenes when subjected to a sequential, one-pot process involving base mediated allylation reactions with ethyl acetoacetate followed by ene-carbocyclization reactions. An unprecedented rearrangement reaction involving 1,5-acyl migration takes place when the methylenecyclopentenes are treated with acid to form highly substituted cyclopentadienes.


 Please wait while we load your content...
Please wait while we load your content...