Identification of pH-dependent synergy on Ru/MoS2 interface: a comparison of alkaline and acidic hydrogen evolution†
Abstract
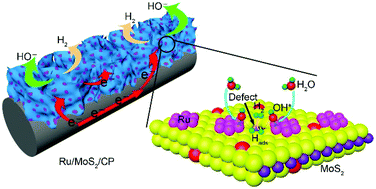
Engineering bifunctional interfaces for enhanced alkaline hydrogen evolution reaction (HER) kinetics is achieved by rational coupling of Ru nanoparticles and defect-rich MoS2 nanosheets via a simple wet-chemical method. Comprehensive material characterizations, especially high-resolution transmission electron microscopy, reveal well-defined interfaces between both components, leading to interfacial synergy whereby Ru expedites water dissociation and nearby defect-rich MoS2 enables favorable hydrogen adsorption for recombination into H2. The designed Ru/MoS2 material demonstrates remarkable catalytic activity towards alkaline HER (−13 mV at −10 mA cm−2) with stable operation after 12 h or 1000 cycles, which is superior to almost all Ru-based and MoS2-based electrocatalysts and even outperforms commercial 20 wt% Pt/C at overpotentials larger than −78 mV in alkaline media. No improved HER activity is observed for Ru/MoS2 in acidic electrolyte (−96 mV at −10 mA cm−2), which is even inferior to Ru/CP (−78 mV at −10 mA cm−2). The correlation between alkaline and acidic HER results confirms that the intrinsic HER activity of this material originates from the desired synergistic effect under alkaline conditions.


 Please wait while we load your content...
Please wait while we load your content...