Controlling the Sn–C bonds content in SnO2@CNTs composite to form in situ pulverized structure for enhanced electrochemical kinetics†
Abstract
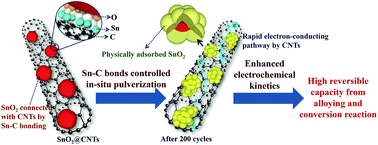
The Sn–C bonding content between the SnO2 and CNTs interface was controlled by the hydrothermal method and subsequent heat treatment. Electrochemical analysis found that the SnO2@CNTs with high Sn–C bonding content exhibited much higher capacity contribution from alloying and conversion reaction compared with the low content of Sn–C bonding even after 200 cycles. The high Sn–C bonding content enabled the SnO2 nanoparticles to stabilize on the CNTs surface, realizing an in situ pulverization process of SnO2. The in situ pulverized structure was beneficial to maintain the close electrochemical contact of the working electrode during the long-term cycling and provide ultrafast transfer paths for lithium ions and electrons, which promoted the alloying and conversion reaction kinetics greatly. Therefore, the SnO2@CNTs composite with high Sn–C bonding content displayed highly reversible alloying and conversion reaction. It is believed that the composite could be used as a reference for design chemically bonded metal oxide/carbon composite anode materials in lithium-ion batteries.


 Please wait while we load your content...
Please wait while we load your content...