Properties of in situ generated gold nanoparticles in the cellular context†
Abstract
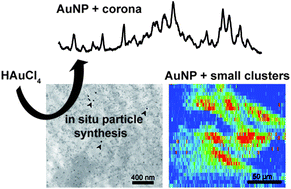
Gold nanostructures that serve as probes for nanospectroscopic analysis of eukaryotic cell cultures can be obtained by the in situ reduction of tetrachloroauric acid (HAuCl4). To understand the formation process of such intracellularly grown particles depending on the incubation medium, the reaction was carried out with 3T3 fibroblast cells in three different incubation media, phosphate buffer, Dulbecco's Modified Eagle Medium (DMEM), and standard cell culture medium (DMEM with fetal calf serum). The size, the optical properties, the biomolecular corona, and the localization of the gold nanoparticles formed in situ vary for the different conditions. The combination of surface-enhanced Raman scattering (SERS) and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) microscopic mapping and transmission electron microscopy (TEM) provides complementary perspectives on plasmonic nanoparticles and non-plasmonic gold compounds inside the cells. While for the incubation with HAuCl4 in PBS, gold particles provide optical signals from the nucleus, the incubation in standard cell culture medium leads to scavenging of the toxic molecules and the formation of spots of high gold concentration in the cytoplasm without formation of SERS-active particles inside the cells. The biomolecular corona of nanoparticles formed in situ after incubation in buffer and DMEM differs, suggesting that different intracellular molecular species serve for reduction and stabilization. Comparison with data obtained from ready-made gold nanoparticles suggests complementary application of in situ and ex situ generated nanostructures for optical probing.


 Please wait while we load your content...
Please wait while we load your content...