Self-assembled air-stable magnesium hydride embedded in 3-D activated carbon for reversible hydrogen storage†
Abstract
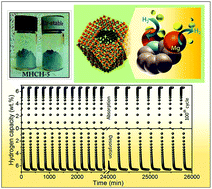
The rational design of stable, inexpensive catalysts with excellent hydrogen dynamics and sorption characteristics under realistic environments for reversible hydrogen storage remains a great challenge. Here, we present a simple and scalable strategy to fabricate a monodispersed, air-stable, magnesium hydride embedded in three-dimensional activated carbon with periodic synchronization of transition metals (MHCH). The high surface area, homogeneous distribution of MgH2 nanoparticles, excellent thermal stability, high energy density, steric confinement by carbon, and robust architecture of the catalyst resulted in a noticeable enhancement of the hydrogen storage performance. The resulting MHCH-5 exhibited outstanding hydrogen storage performance, better than that of most reported Mg-based hydrides, with a high storage density of 6.63 wt% H2, a rapid kinetics loading in <5 min at 180 °C, superior reversibility, and excellent long-term cycling stability over ∼435 h. The significant reduction of the enthalpy and activation energy observed in the MHCH-5 demonstrated enhancement of the kinetics of de-/hydrogenation compared to that of commercial MgH2. The origin of the intrinsic hydrogen thermodynamics was elucidated via solid state 1H NMR. This work presents a readily scaled-up strategy towards the design of realistic catalysts with superior functionality and stability for applications in reversible hydrogen storage, lithium ion batteries, and fuel cells.


 Please wait while we load your content...
Please wait while we load your content...