Integrated hydrogen evolution and water-cleaning via a robust graphene supported noble-metal-free Fe1−xCoxS2 system†
Abstract
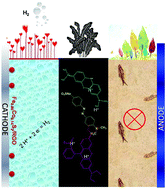
In an electrocatalytic hydrogen evolution reaction (HER) system, a cathodic H+ resource, an anodic sacrificial agent and a robust catalyst are three essential factors. Industry wastewater emissions, containing high levels of acidity and organic dyes, actually can satisfy the material requirements for the HER. Herein, a new HER method is proposed, taking acidic ions from wastewater as a cathodic resource to produce H2, while organic dye waste acts as an anodic sacrifice to balance the reaction. In such a way, a sustainable H2 energy source can be generated and clean water is obtained as well. For the HER catalyst, low cost and highly efficient graphene supported Fe1−xCoxS2 was synthesized with an onset overpotential of ∼50 mV and it demonstrated impressive HER performance in both practical industry wastewater and analogous wastewater simulations. Besides the cathodic H2 evolution, anodic organic dyes (MO, MB, RhB and industry waste organic dyes) were all entirely decomposed within 8 min, 18 min, 9 min and 4 h under oxidation potentials of ∼1.46, 1.50, 1.47 and 1.40 V. As verified both in practical industry wastewater and wastewater simulations in the laboratory, our approach for integrating the HER and wastewater treatment puts forward an attractive opportunity in energy and environmental research fields.


 Please wait while we load your content...
Please wait while we load your content...