Controllable hydrothermal synthesis of Ni/H-BEA with a hierarchical core–shell structure and highly enhanced biomass hydrodeoxygenation performance†
Abstract
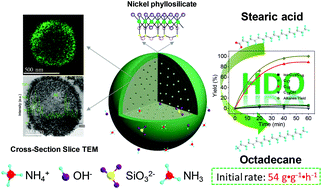
Ni based catalysts are wildly used in catalytic industrial processes due to their low costs and high activities. The design of highly hierarchical core–shell structured Ni/HBEA is achieved using a sustainable, simple, and easy-tunable hydrothermal synthesis approach using combined NH4Cl and NH3·H2O as a co-precipitation agent at 120 °C. Starting from a single-crystalline hierarchical H+-exchanged beta polymorph zeolite (HBEA), the adjustment of the precipitate conditions shows that mixed NH4Cl and NH3·H2O precipitates with proper concentrations are vital in the hydrothermal synthesis for preserving a good crystalline morphology of HBEA and generating abundant highly-dispersed Ni nanoparticles (loading: 41 wt%, 5.9 ± 0.7 nm) encapsulated onto/into the support. NH4Cl solution without an alkali is unable to generate abundant Ni nanoparticles from Ni salts under the hydrothermal conditions, whereas NH3·H2O seriously damages the pore structure. After studying the in situ changes in infrared, X-ray diffractometry, temperature-programmed reduction, and scanning electron microscopy measurements, as well as variations in the filtrate pH, Si/Al ratios, and solid sample Ni loading, a two-step dissolution–recrystallization process is proposed. The process consists of Si dissolution and no change in elemental Al, and after the dissolved Si(IV) concentrations have promoted Ni phyllosilicate nanosheet solubility, further growth of multilayered Ni phyllosilicate nanosheets commences. The precursor Ni phyllosilicate is changeable between Ni3Si2O5(OH)4 and Ni3Si4O10(OH)2, because of competition in kinetically-favored and thermodynamically-controlled species caused by different basic agents. The superior catalytic performance is demonstrated in the metal/acid catalyzed biomass derived bulky stearic acid hydrodeoxygenation with 90% octadecane selectivity and a promising rate of 54 g g−1 h−1, which highly excels the reported rates catalyzed by Ni catalysts. Significant improvements in activity and selectivity are related to the highly dispersive Ni nanoparticles onto/into intra-mesopores of hierarchical HBEA, hence enhance the accessibility of bulky substrates to metal sites and mass transfer capacity.


 Please wait while we load your content...
Please wait while we load your content...