Electrochemical synthesis of nanoporous tungsten carbide and its application as electrocatalysts for photoelectrochemical cells†
Abstract
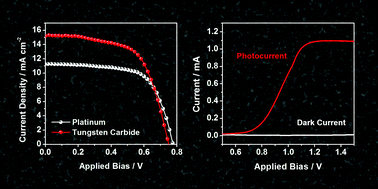
Photoelectrochemical (PEC) cells are promising tools for renewable and sustainable solar energy conversion. Currently, their inadequate performance and high cost of the noble metals used in the electrocatalytic counter electrode have postponed the practical use of PEC cells. In this study, we report the electrochemical synthesis of nanoporous tungsten carbide and its application as a reduction catalyst in PEC cells, namely, dye-sensitized solar cells (DSCs) and PEC water splitting cells, for the first time. The method employed in this study involves the anodization of tungsten foil followed by post heat treatment in a CO atmosphere to produce highly crystalline tungsten carbide film with an interconnected nanostructure. This exhibited high catalytic activity for the reduction of cobalt bipyridine species, which represent state-of-the-art redox couples for DSCs. The performance of tungsten carbide even surpassed that of Pt, and a substantial increase (∼25%) in energy conversion efficiency was achieved when Pt was substituted by tungsten carbide film as the counter electrode. In addition, tungsten carbide displayed decent activity as a catalyst for the hydrogen evolution reaction, suggesting the high feasibility for its utilization as a cathode material for PEC water splitting cells, which was also verified in a two-electrode water photoelectrolyzer.


 Please wait while we load your content...
Please wait while we load your content...