Enhancing coupled enzymatic activity by conjugating one enzyme to a nanoparticle†
Abstract
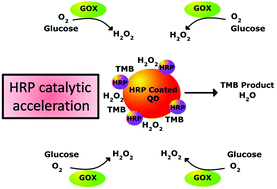
Enzymes have long been a prime research target for the commercial production of commodity and specialty chemicals, design of sensing devices, and the development of therapeutics and new chemical processes. Industrial applications for enzymes can potentially be enhanced by enzyme immobilization which often allows for increased enzyme stability, facile product purification, and minimized substrate diffusion times in multienzymatic cascades, but this is usually at the cost of a significant decrease in catalytic rates. Recently, enzyme immobilization has been advanced by the discovery that nanoparticle surfaces are frequently able to enhance the activity of the bound enzyme. Here we extend this observation to a multienzymatic coupled system using semiconductor quantum dots (QDs) as a model nanoparticle material and the prototypical enzyme pair of glucose oxidase (GOX) and horseradish peroxidase (HRP). We first demonstrate that HRP binding to QDs has a significant beneficial effect on enzymatic activity, producing a >2-fold improvement in kcat. We argue that this enhancement is due to affinity of the QD surface for the substrate. Furthermore, we demonstrate that when the ratio of GOX to HRP is adjusted to allow HRP to be the rate-limiting step of the pathway, the QD-induced rate enhancement of HRP can be maintained in a multi-enzyme cascade. Kinetic analysis shows that the underlying processes can be simulated numerically and provide insight into the governing mechanisms. The potential of nanoparticle-based catalytic enhancement is then discussed in the context of multienzyme cascades and synthetic biology.


 Please wait while we load your content...
Please wait while we load your content...