Order–disorder phase transitions in Au–Cu nanocubes: from nano-thermodynamics to synthesis†
Abstract
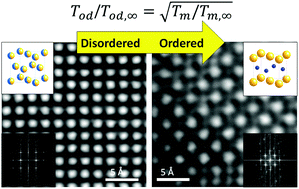
Catalysts have been widely used in industries and can be optimized by tuning the composition and chemical ordering of the elements involved in the nano-alloy. Among bi-metallic alloys, the Au–Cu system is of particular interest because it exhibits ordered phases at low temperatures. Nevertheless, the temperature at which these ordered structures are formed is totally unknown at the nanoscale. Consequently, to speed up the development of these catalysts, this paper theoretically predicts the structural phase transitions between ordered and disordered phases for the Au–Cu system by using nano-thermodynamics. Following the predictions, the suggested annealing temperatures have been carefully chosen and consequently, Au–Cu ordered nanocubes have been successfully synthesized through a solventless protocol. The results are fully supported by electron microscopy observations.


 Please wait while we load your content...
Please wait while we load your content...