Glycine-functionalized copper(ii) hydroxide nanoparticles with high intrinsic superoxide dismutase activity†
Abstract
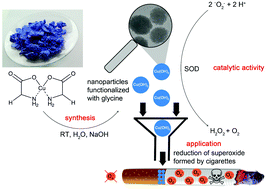
Superoxide dismutases (SOD) are a group of enzymes that catalyze the dismutation of superoxide (O2−) radicals into molecular oxygen (O2) and H2O2 as a first line of defense against oxidative stress. Here, we show that glycine-functionalized copper(II) hydroxide nanoparticles (Gly-Cu(OH)2 NPs) are functional SOD mimics, whereas bulk Cu(OH)2 is insoluble in water and catalytically inactive. In contrast, Gly-Cu(OH)2 NPs form water-dispersible mesocrystals with a SOD-like activity that is larger than that of their natural CuZn enzyme counterpart. Based on this finding, we devised an application where Gly-Cu(OH)2 NPs were incorporated into cigarette filters. Cigarette smoke contains high concentrations of toxic reactive oxygen species (ROS, >1016 molecules per puff) including superoxide and reactive nitrogen species which lead to the development of chronic and degenerative diseases via oxidative damage and subsequent cell death. Embedded in cigarette filters Gly-Cu(OH)2 NPs efficiently removed ROS from smoke, thereby protecting lung cancer cell lines from cytotoxic effects. Their stability, ease of production and versatility make them a powerful tool for a wide range of applications in environmental chemistry, biotechnology and medicine.


 Please wait while we load your content...
Please wait while we load your content...