NiMn layered double hydroxides as efficient electrocatalysts for the oxygen evolution reaction and their application in rechargeable Zn–air batteries†
Abstract
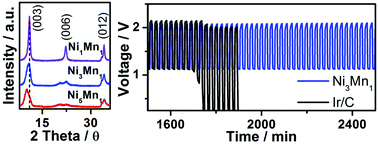
High performance catalysts for the oxygen evolution reaction (OER) are in demand to improve the re-chargeability of Zn–air batteries. In this work, atomically dispersed NiMn layered double hydroxides are prepared via simple hydrothermal synthesis and tested as the OER catalyst in rechargeable Zn–air batteries. NiMn layered double hydroxides with the optimized Ni : Mn molar feeding ratio have good crystallinity, big interlayer spacing, and large surface area, which are beneficial to enhance their catalytic activity. They are highly active and stable during the OER, showing an overpotential of 0.35 V, a Tafel slope of 40 mV dec−1, and remarkable stability during 16 h of a chronopotentiometry test. Rechargeable Zn–air batteries with NiMn layered double hydroxides as the OER catalyst exhibit a low charge voltage of ≈2 V which is stable for up to 200 cycles. This study illustrates a platform to enhance the catalytic activity of the OER catalyst via fine-tuning the composition and physical properties of the materials and their application for rechargeable metal–air batteries.


 Please wait while we load your content...
Please wait while we load your content...