Redox-dependent properties of DTF-endcapped π-oligomers†
Abstract
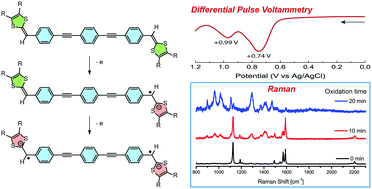
A π-conjugated p-phenylene ethynylene oligomer with redox-active dithiafulvenyl (DTF) groups appended at its two terminal positions was subjected to density functional theory (DFT) calculations and various experimental characterizations using UV-Vis absorption, cyclic voltammetry, and Raman spectroscopy. Collectively, the theoretical and experimental results establish a comprehensive understanding of the structural and electronic properties of the bis(DTF)-functionalized π-oligomer in its neutral and oxidized states. Furthermore, the supramolecular self-assembling properties of this π-oligomer on the surfaces of mica and highly ordered pyrolytic graphite (HOPG) were investigated by atomic force microscopic (AFM) imaging, and the results show that the surface assemblies of the bis(DTF)-oligomer vary significantly in different redox states.


 Please wait while we load your content...
Please wait while we load your content...