Click-on fluorescent triazolyl coumarin peptidomimetics as inhibitors of human breast cancer cell line MCF-7†
Abstract
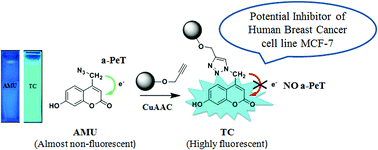
The development of a series of multipurpose triazolyl coumarin peptidomimetics that can also work as click-on fluorescent inhibitors of human breast cancer cell line MCF-7 is described. The quantum mechanical calculations indicate that the click-on fluorescence observed from the azidomethyl coumarin precursors upon click cycloaddition reactions with various alkynes could be due to a decrease in the electron density and an increase in the gap between the HOMO energy levels of the excited coumarin unit and the methyl group upon triazole formation which in turn reduce the process of acceptor photoinduced electron transfer (a-PeT). The tunable and intense click-on fluorescence observed when the coumarin alkynes are replaced with bioisosteric open chain ketoamides points to the possibility of developing a large number of multipurpose probes/anticancer agents through systematic structure alterations in the system.


 Please wait while we load your content...
Please wait while we load your content...