Synthesis and photo-/electro-catalytic properties of Keggin polyoxometalate inorganic–organic hybrid layers based on d10 metal and rigid benzo-diazole/-triazole ligands†
Abstract
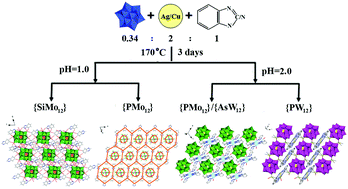
Herein, five Keggin polyanion-templated inorganic–organic hybrids, (H3biz)4[SiMo12O40] (1), (H3biz)3(H2biz)[PMo12O40] (2), [{Cu7(Hbtz)2(btz)4}{PMo12O40}] (3), [{Ag7(Hbtz)2(btz)4}{AsW12O40}] (4), and [{Ag9(H2biz)2(biz)4} {H2PW12O40}] (5) (H2biz = benzimidazole, Hbtz = benzotriazole) were hydrothermally synthesized and characterized via elemental analysis, thermogravimetry (TG), infrared spectroscopy (IR), ultraviolet spectroscopy (UV), and single-crystal X-ray diffraction. A remarkable aspect of compound 1 is that six pairs of H2biz ligands are circularly arranged around the {SiMo12} cluster; this leads to the formation of a unique 2-D supramolecular layer. The most prominent feature of the structure of compound 2 is that protonated Hbiz ligands link the {PMo12} cluster into hexagonal units, which are further extended into a 2-D supramolecular array via edge-sharing modes. The Keggin {PMo12} and {AsW12} clusters in compound 3 and 4 are first extended into octa-connected 2-D sheets by the unusual heptanuclear complex {M7(Hbtz)2(btz)4} (M = Cu for 3 and Ag for 4) linker. Interestingly, two types of M–M interactions exist in the heptanuclear units. Compound 5 exhibits an unprecedented 2D network constructed from nine-nuclear silver complex {Ag9(biz)4(H3biz)2} bridges and {PW12} clusters. Most strikingly, carbene silver and the coexistence of multiple Ag–C bonds are first observed in the nine-nuclear unit. Intensive CuI/AgI–π interactions and π–π stacking play important roles in the packing arrangements of 3–5. Additionally, the electrochemical, electrocatalytic, fluorescence, and photocatalytic properties of 1–5 have been investigated in detail.


 Please wait while we load your content...
Please wait while we load your content...