In situ determination of dissolution kinetics of d-limonene in supercritical carbon dioxide by Raman spectroscopy†
Abstract
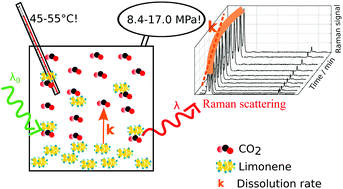
The study of solvation in pressurized systems requires in situ analysis. An optimal spectroscopic technique for such studies would enable the detection of any compound and have resolution enough to differentiate different compounds within a multicomponent mixture. Here we show for the first time that we can follow dissolution kinetics of a model compound, D-limonene, in supercritical carbon dioxide using in situ Raman spectroscopy. Dissolution rate constants were measured at different stirring speeds, temperatures (45 and 55 °C) and amounts of CO2, corresponding to pressures in the range of 8.4 to 17.0 MPa. Dissolution half-lives ranged from 3 min (at 45 °C and 8.4 MPa) up to more than 1 hour (at 55 °C 16.9 MPa). The results indicate that dissolution is mostly controlled by diffusion, while convection did not play a significant role. Dissolution rate constants showed a non-linear inverse relation with diffusivity, while temperature did not influence the dissolution rate constants significantly. Dissolution kinetics is revealed as a significant aspect to consider for the optimization of extraction and separation processes based on supercritical carbon dioxide. Overall, this study offers new insight into solvation phenomena in pressurized fluids.


 Please wait while we load your content...
Please wait while we load your content...