Synthesis of a bicyclic oxo-γ-lactam from a simple caprolactam derivative†
Abstract
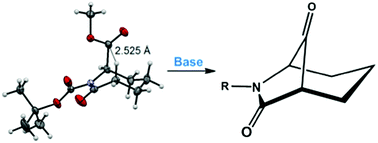
The synthesis of the 6-azabicyclo[3.2.1]octane ring system, via Dieckmann cyclization, is described. Ring closure involves reaction of a caprolactam enolate with a C-6 ester, the reactive axial conformation of which is promoted by the presence of an N-tert-butyloxycarbonyl group on the lactam nitrogen. The results will enable the synthesis of new bridged caprolactams for testing as antibacterials and nucleophilic enzyme inhibitors.


 Please wait while we load your content...
Please wait while we load your content...