Ruthenium arene complexes with triphenylphosphane ligands: cytotoxicity towards pancreatic cancer cells, interaction with model proteins, and effect of ethacrynic acid substitution†
Abstract
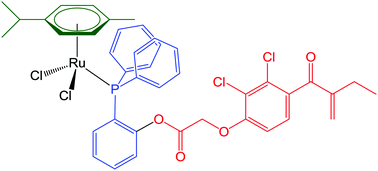
The ruthenium-arene complexes [(η6-p-cymene)RuCl2(κP-PPh2(4-C6H4CO2H))], 1, [(η6-p-cymene)RuCl(κ2P,O-PPh2(2-C6H4CO2))], 2, [(η6-p-cymene)RuCl2(κP-PPh2(2-C6H4OCO-EA))], 3, and [(η6-p-cymene)RuCl2(κP-PPh2(4-C6H4CO2CH2CH2OCO-EA))], 4 (EA-CO2H = ethacrynic acid), were synthesized in good to high yields and characterized by analytical techniques, IR, UV-Vis and multinuclear NMR spectroscopy, and single crystal X-ray diffraction in the cases of 1 and 2. The unstable compounds [(η6-arene)RuCl2(κP-PPh2(2-C6H4CO2CH2CH2OCO-EA))] (arene = p-cymene, 5a; arene = C6H6, 5b) were obtained and identified in solution by NMR. Electrochemical and spectro-electrochemical experiments were performed in order to assess the redox behaviour of 1–4 in CH2Cl2. The in vitro cytotoxicity of 1–4 was determined on the human pancreatic cancer cell line BxPC3 and the mouse embryo fibroblast Balb/3T3 Clone A31 cell line, the latter acting as a model for normal cells. Furthermore, the interaction of 1, 3 and 4 with two model proteins was investigated by high resolution ESI-MS.


 Please wait while we load your content...
Please wait while we load your content...