Reconnaissance of reactivity of an Ag(ii)SO4 one-electron oxidizer towards naphthalene derivatives†
Abstract
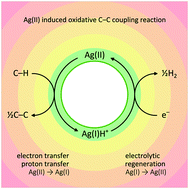
We test divalent silver sulphate, Ag(II)SO4 as a novel reagent for oxidative coupling of aromatic hydrocarbons under ambient temperature conditions. The applicability of the C(sp2)–C(sp2) coupling protocol is illustrated for naphthalene and its 1-substituted derivatives containing either electron donating (e.g. Me, MeO, or Ph) or electron-withdrawing groups (X = F⋯I), leading to 4,4′-disubstituted-1,1′-binaphthyls. Coupling of 2-bromo-naphthalene yields a mixture of 2,2′-, 2,7′-, and 7,7′-dibromo-1,1′-binaphthyls together with their trimeric and tetrameric analogues. The coupling of strongly electron-withdrawing 1-CF3-naphthalene provides the 5,5′-disubstituted-1,1′-binaphthyl derivative. The new method does not require the presence of halogen substituents, in contrast to most of the known C–C coupling methods, and it preserves them, if present. Ag(II)SO4 may be easily electrochemically regenerated from the Ag(I)HSO4 byproduct. However, the C–C coupling method currently suffers from low yields, up to 17%, and it requires further optimization.


 Please wait while we load your content...
Please wait while we load your content...