Retracted Article: Kinetic and mechanistic studies on adsorption of Cu(ii) in aqueous medium onto montmorillonite K10 and its modified derivative†
Abstract
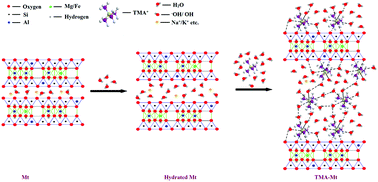
Copper is a widely used metal and known as a micronutrient; however, it is poisonous to the living organisms and has a permissible concentration of 1.3 mg L−1 in aqueous solutions. Thus, it is necessary to provide a suitable, environmentally friendly, and cost-effective copper removal process to save the world. The adsorption technique has been found to be an effective method for the treatment and removal of copper from aqueous solutions. Clays and clay minerals are naturally abundant adsorbents used in this field from very early days. The commercially available montmorillonite K10 clay mineral is modified via intercalation with tetramethylammonium cations to enhance its characteristic properties. The materials are characterized via CEC, FTIR, XRD, TGA-DTG, BET N2-adsorption, and SEM-EDX measurements, and their adsorption capacities for Cu(II) removal from a water system are investigated. Cu(II) adsorption study shows an increase in adsorption capacity from 41.71 to 96.31 mg g−1 after modification of Mt, and at pH 7.0, the Cu(II) removal capacity reaches up to 99.40%. The kinetics study and statistical analysis shows pseudo-second order kinetics as the best fit model for Cu(II) adsorption controlled by more than one mechanism. Thermodynamic study shows the endothermic and spontaneous nature of Cu(II) adsorption with higher affinity towards the TMA-Mt surface. The isotherm study shows the Langmuir maximum adsorption capacity of Mt and TMA-Mt to be 450.45 and 925.93 mg g−1, respectively, for Cu(II) removal from a water system.


 Please wait while we load your content...
Please wait while we load your content...