Theoretical and experimental study of the ground and excited states of 1,4-dihydropyridine based hexahydroquinoline derivatives achieved by microwave irradiation†
Abstract
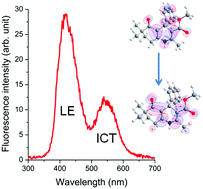
In this article, the photophysical characterisation in solution of a series of hexahydroquinoline derivatives is described using UV-Vis absorption and steady-state fluorescence emission spectroscopies. The compounds were obtained from one-pot four component synthesis via Hantzsch reaction using microwave irradiation. These compounds present absorption maxima located at around 350 nm and fluorescence emission maxima in the UV-violet-blue region. The Lippert–Mataga correlation indicates an ICT character in the excited state for the studied compounds since a linear relation of the fluorescence maxima versus the solvent polarity function was found. Theoretical calculations were also performed in order to study the geometry and charge distribution of these compounds in their ground and excited electronic states. No significant changes in absorption and emission maximum wavelength were found by changing the solvent or substituent groups attached to the hexahydroquinoline structure.


 Please wait while we load your content...
Please wait while we load your content...