Halogen-bonding contacts determining the crystal structure and fluorescence properties of organic salts†
Abstract
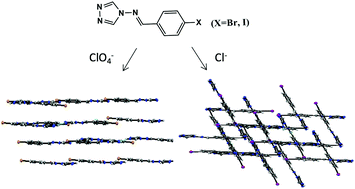
By using the ligands 4-(4-bromobenzylideneamino)-4H-1,2,4-triazole (L1) and 4-(4-iodobenzylideneamino)-4H-1,2,4-triazole (L2), four different organic salts HL1+·Cl− (1), L1·HL1+·ClO4− (2), HL2+·Cl− (3), and L2·HL2+·ClO4− (4) have been synthesized and structurally characterized through single-crystal X-ray diffraction (L1, L2, 2 and 3) and PXRD (1 and 4) measurements. The results revealed that ligands L1 and L2 themselves exhibit a polymeric 1-D chain in a zigzag structure connected by C–Br⋯N and C–I⋯N halogen-bonding contacts, respectively. L1 in salt 2 was connected into a 3-D structure through N–H⋯O hydrogen-bonding contacts, while the interleaved 3-D network of L2 in salt 3 was mainly connected by C–I⋯Cl halogen-bonding contacts. As a result, the fluorescence properties of ligands L1 and L2 were reserved in halogen-bonding connected salts 1 and 3, while there was a reduction in hydrogen-bonding connected salts 2 and 4. Thus, the relationship between emission properties and halogen-bonding contacts can be proposed.


 Please wait while we load your content...
Please wait while we load your content...