Solubility and hydrolysis of Tc(iv) in dilute to concentrated KCl solutions: an extended thermodynamic model for Tc4+–H+–K+–Na+–Mg2+–Ca2+–OH−–Cl−–H2O(l) mixed systems
Abstract
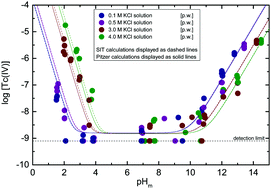
The solubility of 99Tc(IV) under strongly reducing conditions (pe + pHm = 2, with pHm = −log[H+]) is investigated in KCl solutions of different ionic strengths (I = 0.1, 0.5, 3.0 and 4.0 M) at 1.5 ≤ pHm ≤ 14.5. Undersaturation solubility experiments were conducted in an Ar-glovebox (O2 < 1 ppm) at T = (22 ± 2) °C. Liquid–liquid extraction confirmed that the redox state of Tc was kept as +IV during the timeframe of the experiments (≤500 days). Solid phase characterization performed by XRD, SEM-EDS, quantitative chemical analysis and TG-DTA confirmed that TcO2·0.6H2O(s) controls the solubility of Tc(IV) under the conditions investigated. Experimental solubility data determined at 1 ≤ pHm ≤ 10 are properly explained assuming the predominance of Tc3O52+ and TcO(OH)2(aq) in the aqueous phase, and considering thermodynamic and activity models recently reported in the literature for these species. Above pHm ≈ 10, the combination of solid phase characterization with slope analysis of solubility data indicates a solubility control by the chemical reaction TcO2·0.6H2O(s) + 1.4H2O(l) ⇔ TcO(OH)3− + H+. The hydrolysis constant and SIT/Pitzer ion interaction coefficients of TcO(OH)3− with K+ have been determined in the present work. Additional solubility experiments with TcO2·0.6H2O(s) in mixed alkaline KCl–NaCl–MgCl2–CaCl2 electrolyte solutions of specific relevance in the context of nuclear waste disposal show excellent agreement with thermodynamic calculations using thermodynamic and activity models derived in our group for the system Tc4+–H+–K+–Na+–Mg2+–Ca2+–OH−–Cl−–H2O(l).


 Please wait while we load your content...
Please wait while we load your content...