The effect of chalcogen substitution on the structure and spectroscopy of 4,7-dimethyl-2H-chromen-2-one/thione analogues†
Abstract
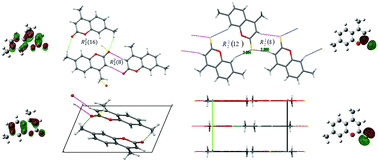
4,7-Dimethyl-2H-chromen-2-one (1) and its novel sulfur analogue 4,7-dimethyl-2H-chromen-2-thione (2) were synthesized and fully characterized by spectroscopic methods and mass spectrometry. The crystal structure of compound 2 has been determined by X-ray diffraction methods, and it crystallizes in the monoclinic I2/m space group. The molecular skeleton lies in the crystallographic mirror plane, in agreement with the expected theoretical planarity enforced by extended π-bonding. The crystal packing of both compounds is characterized by the R22(8) motif formed by intermolecular C–H⋯X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (X = O and S) hydrogen-bonding interactions involving the 1-benzopyran-2-one/thione moiety, as also identified by Hirshfeld surface analysis. The vibrational properties have been studied by infrared and Raman spectroscopy complemented by quantum chemical calculations and normal coordinate analysis of the potential energy distribution. The UV-Vis spectrum is interpreted in terms of TD-DFT quantum chemical calculations, showing a clear red-shift in the HOMO–LUMO electronic transition when the chromen-2-one ring is converted into chromen-2-thione.
C (X = O and S) hydrogen-bonding interactions involving the 1-benzopyran-2-one/thione moiety, as also identified by Hirshfeld surface analysis. The vibrational properties have been studied by infrared and Raman spectroscopy complemented by quantum chemical calculations and normal coordinate analysis of the potential energy distribution. The UV-Vis spectrum is interpreted in terms of TD-DFT quantum chemical calculations, showing a clear red-shift in the HOMO–LUMO electronic transition when the chromen-2-one ring is converted into chromen-2-thione.


 Please wait while we load your content...
Please wait while we load your content...