Effects of different proton donor and acceptor groups on excited-state intramolecular proton transfers of amino-type and hydroxy-type hydrogen-bonding molecules: theoretical insights†
Abstract
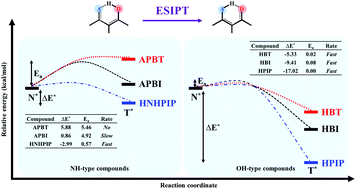
The effect of proton donors, namely NH-type and OH-type, on the excited-state intramolecular proton transfer (ESIPT) of hydrogen-bonding (H-bond) molecules was investigated using density functional theory (DFT) and time-dependent DFT (TD-DFT) at the B3LYP level with the TZVP basis set. The important parameters for bond distances involved in the intramolecular H-bond revealed that H-bonds of OH-type are stronger than those of NH-type and this was supported by the greater red-shift of O–H vibrational modes in the excited-state. The potential energy surfaces along the proton transfer (PT) reaction show that the ESIPT of O–H type occurs with a small barrier or barrierless in the excited-state whereas those of N–H type have higher PT barriers except for the one with a stronger proton acceptor, resulting in a smaller barrier. On-the-fly dynamic simulations on the first excited-state were further carried out to provide the important dynamic information on PT time and probability. The results of dynamic simulations are in accordance with the potential energy surfaces in which the N–H type shows no PT in APBT but slow PT in APBI and fast PT in HNHPIP, while the O–H type (HBI, HBT and HPIP) exhibits ultrafast PT within 80 fs. Moreover, the occurrence of the ESIPT process is strongly dependent on reaction energy and activation energy, in which the H-bond molecules with thermodynamically and kinetically favorable characters always provide the ESIPT. Therefore, the type of proton donor and proton acceptor of H-bond molecules is very important to hinder or effectively facilitate the ESIPT process.


 Please wait while we load your content...
Please wait while we load your content...