One-pot three-component protocol for the synthesis of indolyl-4H-chromene-3-carboxamides as antioxidant and antibacterial agents†
Abstract
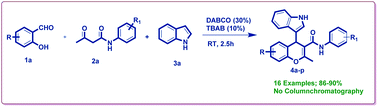
A series of newly synthesized 4-(1H-indol-3-yl)-2-methyl-N-phenyl-4H-chromene-3-carboxamide derivatives were achieved by one-pot reaction between salicylaldehydes, substituted acetoacetanilides, and indoles in methanol catalyzed by 1,4-diazabicyclo [2.2.2]octane (DABCO) (30 mol%) at room temperature. These chromene systems were constructed through Knoevenagel condensation followed by a nucleophilic substitution process. The valuable features of this protocol such as short reaction time, simple operational procedure, broad substrate scope, and high yield of products make it an efficient and promising synthetic strategy. For the first time, various substituted 4H-chromene-3-carboxamide derivatives using DABCO as a catalyst are reported. The synthesized compounds (4a–p) were evaluated in antioxidant and antibacterial studies. The derivatives 4c, 4d, 4k, 4l, and 4p showed good antioxidant activity. Among all the derivatives 4k, 4l, and 4p were found to be active against bacterial strains with MIC values ranging from 9.3 to 18.75 μg mL−1.


 Please wait while we load your content...
Please wait while we load your content...