Sn(ii)-Mediated facile approach for the synthesis of 2-aryl-2H-indazole-3-phosphonates and their anticancer activities†
Abstract
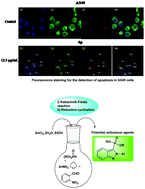
A convenient three-component method was developed for the synthesis of various 2-aryl-2H-indazole-3-phosphonates using SnCl2·2H2O under conventional heating techniques. The transition metal free reaction, mild reaction conditions, one-pot operation, open flask conditions, wide substrate scope and good yields are a few important features of this methodology. This protocol proceeds with high atom economy via the formation of α-aminophosphonates followed by the generation of an indazole ring through N–N bond formation, eliminating water as a by-product. The cytotoxic properties of the 2-aryl-2H-indazole-3-phosphonate derivatives were investigated against A549 and HepG-2 cells. Interestingly, compounds 4d and 4g showed potent cytotoxic properties against HepG-2 cells and compound 4p showed significant cytotoxic properties against A549 cells. Intracellular visualization was done using a laser scanning confocal microscope. DNA fragmentation, colony formation, and apoptosis studies, flow cytometry and western blot analysis for 4p against A549 cells have also been reported.


 Please wait while we load your content...
Please wait while we load your content...