Photosensitized oxidation of 2′-deoxyguanosine 5′-monophosphate: mechanism of the competitive reactions and product characterization
Abstract
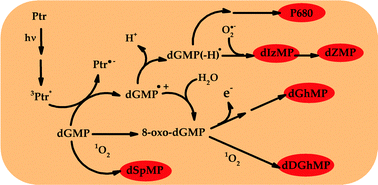
UV-A radiation (320–400 nm) induces modifications to different biomolecules through photosensitized reactions. Oxidized pterins are efficient photosensitizers that accumulate in the skin affected by vitiligo, and photoinduce the oxidation of guanine in a process initiated by an electron transfer from the nucleobase to the triplet excited state of the photosensitizer. In this work, we have investigated the degradation of 2′-deoxyguanosine 5′-monophosphate (dGMP) photosensitized by pterin (Ptr), the parent compound of oxidized pterins, in aqueous solutions under UV-A irradiation. We have identified five products containing the oxidized guanine moiety: the deoxyribonucleoside 5′-monophosphate derivatives of imidazolone, dehydroguanidinohydantoin, guanidinohydantoin, oxazolone and spiroiminodihydantoin. An additional product with a much higher molecular weight, denoted P680, was also detected. The MS/MS analyses show that this compound contains an intact guanine moiety and a modified one. The dependence of the rate of product formation in different experimental conditions was analyzed and a general mechanistic scheme is proposed.


 Please wait while we load your content...
Please wait while we load your content...