Aggregation-induced emission enhancement and aggregation-induced circular dichroism of chiral pentaphenylpyrrole derivatives and their helical self-assembly†
Abstract
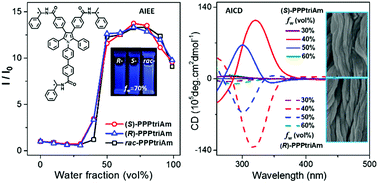
Herein, a pair of enantiomers, (S)-PPPtriAm and (R)-PPPtriAm, with three chiral substituents and their raceme rac-PPPtriAm derived from conjugated luminogen pentaphenylpyrrole were prepared by covalently attaching (R)-/(S)- or racemic 1-phenylethylamine to conjugated 1-biphenyl-2,3,4,5-tetraphenyl pyrrole. The compounds exhibit obvious aggregation-induced emission enhancement (AIEE) features, and the chiral substituents have little effect on the photophysical properties. More importantly, the introduction of chiral substituents endows the chiral compounds (S)-PPPtriAm and (R)-PPPtriAm with distinct aggregation-induced circular dichroism (AICD) with mirror-image Cotton effects and chiral-polarized luminescence (CPL) properties with an emission dissymmetry factor (gem) in the range of 0.5 × 10−3 to 5 × 10−3 for (S)-PPPtriAm and −1 × 10−3 to −6 × 10−3 for (R)-PPPtriAm in a DMSO–water mixture with the water fraction of 40%. The chiral compounds (S)-PPPtriAm and (R)-PPPtriAm can self-assemble into nanofibers, and the lank nanofibers orderly weave and align together to form a regular arrangement of aggregates. The raceme rac-PPPtriAm exhibits AIEE features without any AICD and CPL signals and can self-assemble to form nanoparticle blocks. The enantiomers (S)-PPPtriAm and (R)-PPPtriAm, as AIEE-active luminogens with a multichiral substituent, are expected to have potential applications in photoelectronic materials or in the design of optically active fluorophores for sensitive enantiomer recognition.


 Please wait while we load your content...
Please wait while we load your content...