Hypochlorite-promoted inhibition of photo-induced electron transfer in phenothiazine–borondipyrromethene donor–acceptor dyad: a cost-effective and metal-free “turn-on” fluorescent chemosensor for hypochlorite†
Abstract
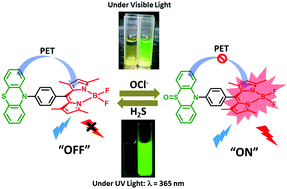
A phenothiazine (PTZ)–borondipyrromethene (BODIPY) based electron donor–acceptor dyad, 1, was designed and used for the sensitive and selective detection of hypochlorite. Upon excitation of the BODIPY moiety, the fluorescence of 1 was observed to be quenched (∼90%) due to photo-induced electron transfer from PTZ to 1BODIPY*. However, upon the addition of incremental amounts of NaOCl, the fluorescence of 1 was enhanced, indicating inhibition of the reductive PET process from PTZ to BODIPY, which was quickly restored upon treatment with H2S, indicating the reversibility of the probe for the continuous detection of hypochlorite. Parallel time-resolved fluorescence studies showed a decrease in the lifetime of the BODIPY moiety compared to the pristine BODIPY, and upon the addition of NaOCl, the lifetime of the BODIPY moiety (0.7 ns) in 1 was observed to be recovered highlighting the real-time applications of the probe in sensing NaOCl. Steady-state fluorescence experiments at varied pH have suggested that 1 can detect NaOCl in the pH range of 7–8, similar to physiological conditions, and at a pH of 7.6, 1 displayed high selectivity for hypochlorite over other reactive oxygen species. To understand the chemical and photophysical mechanisms involved in the fluorescence “turn-on” event, 1 was treated with NaOCl and subjected to ESI-MS analysis. Mass spectrometry showed the formation of oxidized products, sulfoxide and sulfone, and the density functional theory studies confirmed that the formation of these products was responsible for the inhibition of the reductive PET process from the PTZ group to the 1BODIPY* moiety. Also, 1 exhibited enhanced fluorescence upon treatment with commercially available sodium hypochlorite disinfectants, indicating the applications of the probe to real samples. Cytotoxicity studies performed on HEK 293 cells suggested that, up to a 5 μM concentration of 1, the cell viability is above 80%, implicating the applications of the probe for biological samples.


 Please wait while we load your content...
Please wait while we load your content...