Synthesis and photophysical characterization of bacteriochlorins equipped with integral swallowtail substituents†
Abstract
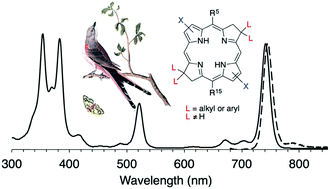
Bacteriochlorins such as bacteriochlorophyll a absorb strongly in the near-infrared spectral region and are potentially useful in a variety of photochemical fields. De novo syntheses of bacteriochlorins entail self-condensation of a dihydrodipyrrin-acetal (containing one pyrrole and one pyrroline joined via a methylidene bridge) either via a heavily studied Eastern–Western (E–W) route or a recently reported Northern–Southern (N–S) route. The Michael addition to form the dihydrodipyrrin-acetal for the E–W approach has limited scope for the installation of substituents on the pyrroline units. By use of the N–S route, new bacteriochlorins have been prepared that bear a pair of aryl or alkyl groups, together termed a “swallowtail” substituent, at each β-pyrroline unit, a previously inaccessible design. Single-crystal X-ray structures of three intermediates were determined. Bacteriochlorins synthesized herein exhibit characteristic bacteriochlorophyll-like absorption spectra, including a Qy band in the region of 730–758 nm. The swallowtail groups have little impact on the excited-state properties of the bacteriochlorins, and the slight changes of spectral properties that are observed stem from substituent electronic effects rather than changes in structure. In summary, introduction of an integral swallowtail unit on the pyrroline ring opens new sites for tailoring molecular designs without altering the attractive photophysical features of the synthetic bacteriochlorins.


 Please wait while we load your content...
Please wait while we load your content...