Synthesis and catalytic applications of palladium N-heterocyclic carbene complexes as efficient pre-catalysts for Suzuki–Miyaura and Sonogashira coupling reactions†
Abstract
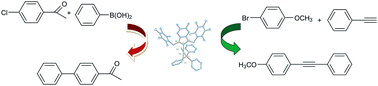
A new palladium complex series with N-heterocyclic carbene (NHC), pyridine and phosphine ligands, PdCl2(L)NHC (2a–c)(L = NHC), PdCl2(L1)NHC(3a–c)(L1 = pyridine), PdCl2(L2)NHC(4a–c)(L2 = triphenylphosphine) was synthesised and fully characterized. The catalytic activities of these complexes were screened for the Sonogashira and Suzuki–Miyaura reactions between arylhalides and phenylacetylene, and phenylboronic acid, respectively. The results pointed out that the carbene/phosphine complexes 4a–c exhibited excellent catalytic activities as compared to 2a–c, 3a–c, and the well-known systems for the palladium-catalysed Sonogashira reaction. The reactivity of 4a–c in these preliminary Sonogashira coupling tests seems to be higher than that of previously reported catalytic systems based on Pd(NHC) moieties. These new palladium NHC complexes are among the first reported palladium catalysts that are efficient for catalysing the Sonogashira reaction from arylchloride substrates.


 Please wait while we load your content...
Please wait while we load your content...