Synthesis, structural studies and stability of model cysteine containing DNA–protein cross-links†
Abstract
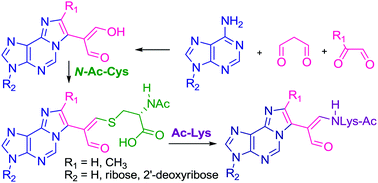
DNA–protein cross-links (DPCs) are bulky, helix-distorting lesions that are formed upon irreversible bonding of proteins to chromosomal DNA in the presence of cross-linking agents. Among a broad range of such agents are α,β-unsaturated carbonyl compounds, which act essentially as bifunctional alkylating agents and form adducts with DNA bases. These adducts can further undergo interactions with other cellular macromolecules leading to the formation of cross-linked products. We synthesized and structurally characterized N-acetylcysteine cross-links formed in the reactions with aldehydic adducts of adenine nucleosides, which possess enol functionality and represent model α,β-unsaturated carbonyl systems. Studies performed by the use of NMR spectroscopy, DFT and ab initio methods established that two of these cross-links exist as rotamers stable at room temperature. Application of Atoms in Molecules Theory enabled hydrogen bonding and other stabilizing interactions within the studied molecules to be estimated. Under physiological conditions the cross-links were found to be relatively stable until Nα-acetyllysine was present in the reaction medium. The presence of this amino acid caused fast transformation of the N-acetylcysteine cross-links into a range of their lysine derivatives. Although instability of the cysteine adduct with acrolein was reported, we showed that the mechanism involved in the gradual decomposition of the N-acetylcysteine cross-links differs from that proposed for acrolein adduct degradation. This demonstrates that in spite of similarities in their structures, numerous α,β-unsaturated carbonyl compounds can interact with nucleophilic biomolecules by different mechanisms leading to structural heterogeneity of the resulting products. Our findings provide an explanation for difficulties in identifying the cysteine containing DPCs in vivo and in vitro, and may be of great importance with respect to detection and isolation of such lesions from biological materials.

 Please wait while we load your content...
Please wait while we load your content...