An efficient Friedel–Crafts alkylation for the synthesis of 3-indolyl-3-hydroxy oxindoles and unsymmetrical 3,3-diaryl oxindoles catalyzed by Dabco-based ionic liquids in water†
Abstract
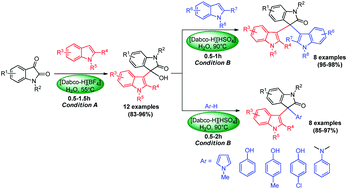
A convenient and rapid method for the syntheses of 3-indolyl-3-hydroxy oxindoles and unsymmetrical 3,3-diaryl oxindoles has been developed by using Dabco-based ionic liquid catalysts. The two ionic liquid catalysts, [Dabco-H][BF4] and [Dabco-H][HSO4] were found to be highly efficient catalysts for controlled 3-indolylation of isatins in water. When [Dabco-H][BF4] was employed as the catalyst in water at 55 °C, the reaction between isatins and indoles stops at the step of addition of the two components and afforded 3-indolyl-3-hydroxy oxindoles. While increasing the reaction temperature to 90 °C, the catalyst [Dabco-H][HSO4] could drive the reaction further and afford symmetrical 3,3-diindolyl oxindoles. By using the two kinds of ionic liquids, a two-step protocol for the efficient synthesis of unsymmetrical 3,3-diaryl oxindoles has also been developed. The use of water as the reaction medium makes the process environmentally benign. The catalysts can be recycled five times without activity loss.


 Please wait while we load your content...
Please wait while we load your content...