The structure and magnetism of mono- and di-nuclear Ni(ii) complexes derived from {N3O}-donor Schiff base ligands†
Abstract
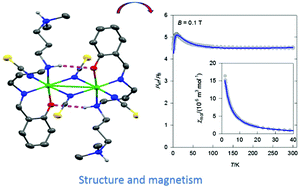
By Schiff condensation, a new tetradentate {N3O}-donor ligand has been prepared in free form. It was combined with Ni(II) salts to yield mononuclear and dinuclear Ni(II) complexes whose structures were determined by single crystal X-ray structure analyses. The magnetic properties reveal some zero-field splitting for two mononuclear complexes and exchange coupling of ferromagnetic nature mediated by (μ1,1-N3), (μ1,1-NCO) and (μ1,1-NCS) bridging coligands. Most importantly, the (μ1,1-NCO) and (μ1,1-NCS) bridging complexes are not only rare dinickel(II) complexes, but also the (μ1,1-NCS) bridging analogue is the first example of a ferromagnetically coupled dinickel(II) system.


 Please wait while we load your content...
Please wait while we load your content...