9-Fluorenone and 9,10-anthraquinone potential fused aromatic building blocks to synthesize electron acceptors for organic solar cells†
Abstract
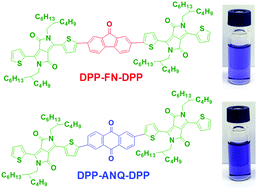
In this work, for the first time we used two novel fused aromatic conjugated electron withdrawing moieties 9-fluorenone and 9,10-anthraquinone, respectively, to design two non-fullerene acceptors and evaluated their viability in solution-processable organic solar cells (OSCs). 9-Fluorenone and 9,10-anthraquinone were used as core electron withdrawing blocks in combination with another common strong electron accepting diketopyrrolopyrrole (DPP) end-capping group. The compounds 6,6′-(5,5′-(9-oxo-9H-fluorene-2,7-diyl)bis(thiophene-5,2-diyl))bis(2,5-bis(2-butyloctyl)-3-(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione) (DPP-FN-DPP) and 6,6′-(5,5′-(9,10-dioxo-9,10-dihydroanthracene-2,6-diyl)bis(thiophene-5,2-diyl))bis(2,5-bis(2-butyloctyl)-3-(thiophen-2-yl)pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione) (DPP-ANQ-DPP) were synthesized via a Suzuki coupling reaction and characterized completely. The new acceptors exhibit good solubility in common organic solvents and good thermal stability with 5% weight loss above 360 °C. DPP-FN-DPP and DPP-ANQ-DPP possess a broad absorption band at 300–700 nm with optical band-gaps of 1.75 and 1.71 eV, respectively. The use of different core acceptor building blocks resulted in a difference in LUMO and HOMO energy levels. Inverted OSC devices employing P3HT as the donor polymer and DPP-FN-DPP and DPP-ANQ-DPP as acceptors yielded quite high open-circuit voltages (VOC) of 0.85–0.98 V, benefiting from the relatively low-lying LUMO energy levels of the two acceptors. Among both, OSC devices based on DPP-FN-DPP as acceptor exhibits the highest performance with a VOC of 0.97 V, a short-circuit current density (JSC) of 3.2 mA cm−2, a fill factor (FF) of 37%, and an overall power conversion efficiency of 1.2%.


 Please wait while we load your content...
Please wait while we load your content...