Nerve agent simulant diethyl chlorophosphate detection using a cyclization reaction approach with high stokes shift system†
Abstract
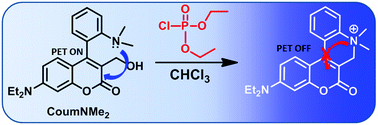
A reaction-based fluorescent probe (CoumNMe2) containing a coumarin-4-dimethyaminoaryl scaffold for the detection of nerve agent simulants was developed. The probe showed fluorescence enhancement selectively with diethyl chlorophosphate (DCP) over close competitors diethyl cyanophosphonate (DECP), and diethyl methylphosphonate (DEMP) with very little interference from metal ions. O–P bond formation by reaction of the benzyl alcohol motif on the probe with DCP, favours intramolecular cyclization that leads to the ammonium salt. The cyclization strongly inhibits the photo-induced electron transfer (PET) process, which leads to the enhancement of fluorescence intensity (∼10 fold). Also, DFT/TDDFT calculations were exploited to explain the nature of the fluorescence “turn-on” process. Herein, we report a new fluorescent probe (CoumNMe2) based on the intramolecular cyclization reaction for the detection of nerve agent simulant and has potential for real applications.


 Please wait while we load your content...
Please wait while we load your content...