Synthesis and study of the stability of amidinium/guanidinium carbamates of amines and α-amino acids†
Abstract
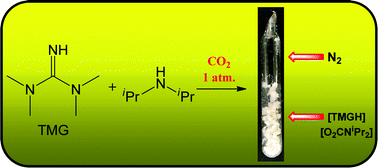
Thermally stable amidinium/guanidinium N,N-dialkylcarbamates, including vacuum stable compounds, have been prepared, and then isolated in the solid state, by reaction of tetramethylguanidine (TMG) or 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) with secondary amines under atmospheric pressure of CO2. The same method has been successfully applied to α-amino acids, thus the corresponding carbamates of sarcosine, L-proline and L-phenylalanine have been obtained. All the products are highly moisture sensitive, and have been characterized by analytical and spectroscopic (IR, multinuclear NMR) techniques.


 Please wait while we load your content...
Please wait while we load your content...