Synthesis and structural characterization of mixed halide–N,N-diethylcarbamates of group 4 metals, including a case of unusual tetrahydrofuran activation†
Abstract
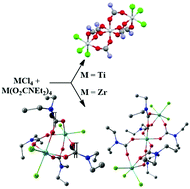
Solid-state, thermally stable tetramethylguanidinium N,N-diethylcarbamate [TMGH][O2CNEt2] was prepared from tetramethylguanidine (TMG), N,N-diethylamine and carbon dioxide (1 atm). The reaction of [TMGH][O2CNEt2] with TiCl4 in toluene afforded [TMGH]2[TiCl6] and TiCl2(O2CNEt2)2. The tetrachlorides of titanium, zirconium and hafnium were effectively converted into the respective mixed dichloride-dicarbamates, MCl2(O2CNEt2)2, by the reaction with either silver diethylcarbamate or the corresponding homoleptic metal N,N-diethylcarbamate, M(O2CNEt2)4. The titanium and hafnium derivatives are trinuclear in the solid state according to X-ray diffraction analysis (M = Ti) and DFT calculations (M = Hf). Instead, ZrCl2(O2CNEt2)4 was obtained as a mixture of two isomers presumably holding dinuclear and trinuclear structures, according to DFT calculations. Difluoride-dicarbamate TiF2(O2CNEt2)4 was prepared in a similar way as the homologous Cl-compound. All the products were characterized by analytical and spectroscopic methods, and by X-ray diffraction in the cases of [TMGH]2[TiCl6] and Ti3Cl6(μ-O2CNEt2)6. The complex Hf4Cl4(O)2(O2CNEt2)6[O(CH2)3CHO]2 was isolated in low yield in crystalline form from a thf solution of HfCl2(O2CNEt2)2, and then X-ray characterized.


 Please wait while we load your content...
Please wait while we load your content...