Conformational change in the association of a heterocyclic urea derivative forming two intramolecular hydrogen bonds in polar solvent†
Abstract
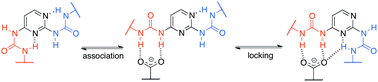
The association of a model, heterocyclic compound capable of forming two intramolecular hydrogen bonds was studied with the use of various anionic and neutral species in highly polar solvents, but also, for some of them, in chloroform. The hydrogen bonding of anions was tuned through the substituents present in their structures. This approach was used in distinguishing which part of the bisurea heterocyclic derivative is preferred during complex formation. Neutral counterparts capable of forming three or five hydrogen bonds were also used. Moreover, triple association was probed, suggesting the formation of a complex only in chloroform. DFT computations were helpful in the interpretation of the experimental data related to complicated equilibria. These are based on the energy of rotation about single bonds, energy of interaction and QTAIM-based energies of hydrogen bonds.


 Please wait while we load your content...
Please wait while we load your content...