Towards a global greener process: from solvent-less synthesis of molybdenum(vi) ONO Schiff base complexes to catalyzed olefin epoxidation under organic-solvent-free conditions†
Abstract
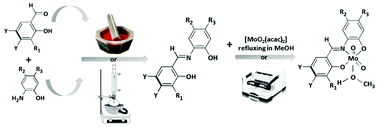
Nine Schiff base ligands derived from o-hydroxyaldehydes (2-hydroxybenzaldehyde, 2-hydroxy-3-methoxybenzaldehyde, 2-hydroxy- 1-naphthaldehyde) and nine corresponding dioxomolybdenum(VI) complexes, cis-[MoO2L(CH3OH)] or cis-[MoO2L(CH3OH)]·CH3OH and dinuclear [MoO2L]2, have been prepared using the conventional solution-based method as well as mechanochemically, by liquid assisted grinding (LAG). All products have been characterised by means of IR spectroscopy, thermal analyses and also by powder and five molybdenum complexes by single crystal X-ray diffraction. The crystal structure analysis of mononuclear complexes reveal distorted octahedral Mo(VI) coordination by ONO donor atoms from a dianionic tridentate Schiff base ligand, two oxido oxygen atoms from the MoO22+ moiety and an oxygen atom from the MeOH molecule trans to the oxido oxygen atom. Due to the trans effect of the oxido oxygen atom, Mo–O(MeOH) is the longest bond distance within the Mo coordination sphere and it expected to be the point of maximum reactivity of the complexes. All complexes have been studied as pre(catalysts) for the epoxidation of cis-cyclooctene, cyclohexene and (R)-limonene using aqueous tert-butyl peroxide (TBHP) as the oxidant and in the absence of an organic solvent.


 Please wait while we load your content...
Please wait while we load your content...