Tuning of synthesis conditions by thermal decomposition towards gadolinium-doped manganese carbonate nanoparticles with uniform size and high relaxivity†
Abstract
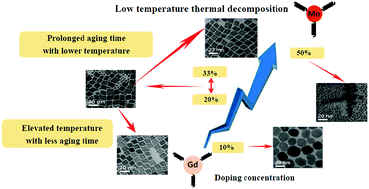
Gd-doped MnCO3 nanoparticles have been prepared by thermal decomposition of Mn-oleate with 20 mol% Gd-oleate at 310 °C. However, MnCO3 tends to transform into MnO at a temperature above 300 °C. Developing thermal decomposition below 300 °C for the synthesis of MnCO3 nanoparticles is therefore highly desirable. Herein, we tested the feasibility of the synthesis of Gd-doped MnCO3 nanoparticles by the thermal decomposition method at a low temperature of 280–300 °C and explored the effects of several parameters on the phase and uniformity of particles. It was revealed that the Gd-doped MnCO3 nanoparticles could be obtained only when the Gd doping concentration is within a range of 20–33 mol%, while the uniformity of the resulting Gd-doped MnCO3 nanoparticles could be improved either by elevating the reaction temperature or prolonging the aging time. In addition, the formation mechanism of MnCO3 nanoparticles with Gd doping was tentatively proposed based on the information acquired by thermogravimetric-gas chromatography-mass spectrometry. Moreover, the obtained uniform-sized Gd-doped MnCO3 nanoparticles present high relaxivity and notable positive contrast enhancement towards brain glioma in mice.

 Please wait while we load your content...
Please wait while we load your content...