Pd-catalyzed intramolecular sequential Heck cyclization and oxidation reactions: a facile pathway for the synthesis of substituted cycloheptenone evaluated using computational studies†
Abstract
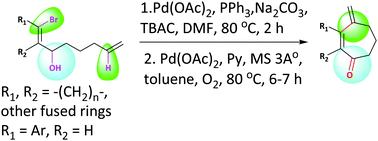
A simple and convenient method for the construction of substituted cycloheptenones from 1-bromoocta-1,7-diene-3-ols has been developed. The reaction involves Pd(0)-catalyzed intramolecular 7-exo-trig cyclization followed by Pd(II)-catalyzed oxidation of cyclic alcohol. The course of the reaction pathway has been evaluated using DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...